Esterification, neutralization and return of flavor in oils
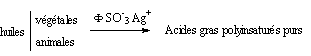
The direct use of vegetable oils as fuel is studied through the neutralization of the crude oil with methanol or with ethanol according to a technique using ion exchange resins as a catalyst. Thanks to a good knowledge of the reaction mechanism involved, it was possible to reach a residual acidity of less than 0.4%.
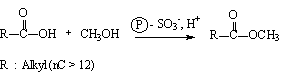
Esterification of the free fatty acids is carried out without degradation of the triglycerides. The product obtained can be partially transesterified or used in a mixture with diesel fuel to give a good diesel fuel.
This process is particularly well suited to highly acidic oils that are unusable for agri-food purposes, which therefore find an original value.
The reactivity of secondary or tertiary alcohols with various anhydrides has been studied. The activation energy required for the evolution of the reaction is provided either by conventional thermal sources or with the aid of electromagnetic anodes.
The application of this process to the esterification of macromolecules should then open a new field of valorization of these compounds.
The study of the high-temperature flavor return of rapeseed oil has made it possible, for the first time, to characterize and separate some residual triterpenes in refined oil, which is partly responsible for this phenomenon. this is substantially the commercial quality of this oil.
Acides gras purs par hydrolyse enzymatique et séparations chromatographiques d’acides gras polyinsaturés
Two routes for obtaining pure fatty acids have been envisaged: the hydrolysis of triglycerides followed by the separation of fatty acids or the hydrolysis of the pure fatty acid esters obtained by the process described above.
Enzymatic catalysis was chosen because it allows mild hydrolysis conditions (low temperature, atmospheric pressure, etc.), unlike chemical catalysis.
Among the enzymes tested, Candida cylindracae and Mucor miehi lipases were retained for their important activity on triglycerides and fatty acid esters. The study of the influence of the various parameters involved on the hydrolysis rate has made it possible to define the optimal conditions of conversion.
The ion exchange resins used as chromatographic medium, with suitable counter-ions, allow the separation of the fatty acid methyl esters from the pastel oil (Isatis tinctoria), as part of a research program on the refining pastel and its possible reintegration into the agricultural landscape of our region.
The operation results in the separation, under large-scale extrapolatable conditions, of linolenic acid thanks to the very surprising difference in the behavior of the complexes formed with the Ag + ions retained by the resins, depending on whether the elution is carried out by methanol or ethanol. The operating procedure was then generalized with equal success to oils containing linolenic acid.
A process for the separation of polyunsaturated fatty acid esters, comprising 3 steps, has been developped :
- a stage of neutralization of the oil by esterification in situ of the free fatty acids,
- a transesterification step with methanol (or ethanol),
- a step of separating the methyl ester mixture obtained on an ion exchange resin in Ag + form based essentially on the ability of Ag + ions to form complexes with the double bonds of unsaturated fatty acid esters. The elution solvents are chosen according to their polarity and their affinity to break the complexes formed between Ag + and methyl linoleate on the one hand and methyl linolenate on the other hand.
The operating protocol was then generalized, with equal success to different oils containing linoleic or linolenic acid. The optimization of the parameters involved during the separation and a modeling of the process led to the realization of an automated and computer controlled pilot unit.
Cruciferous oils, Amazonian palms and other oleaginous oils.
The fixed oils of pastel seeds (Isatis Tinctoria) but also Cynara Cardunculus, Lepidim sativum L, Diplotaxis temuisilic Delile, Eruca sativa, and Sinapis arvensis were studied.
The analysis of the hexanic extracts and solid residues made it possible to characterize the oil of these seeds, by their composition in fatty acid, to identify by mass spectrometry the various characteristic steroids. The content of residual tocopherols and their nature have also been studied.
The content of cellulose, hemicelluloses and lignins of the solid fraction was determined as well as the quantity and the nature of the glucosinolates present obtained by hydroalcoholic extraction.
A similar work was done on a family of Amazonian palms and qualified the composition of the oil of 8 palms which have in common to be much less saturated than that of Eleais Guinensis developed in all tropical latitudes.
The study of the pharmacological properties of the unsaponifiable part of various oils, notably that of awara (astrocaryum vulgare), was carried out.